Gallium Ga (Element 31) of Periodic Table Element FlashCards
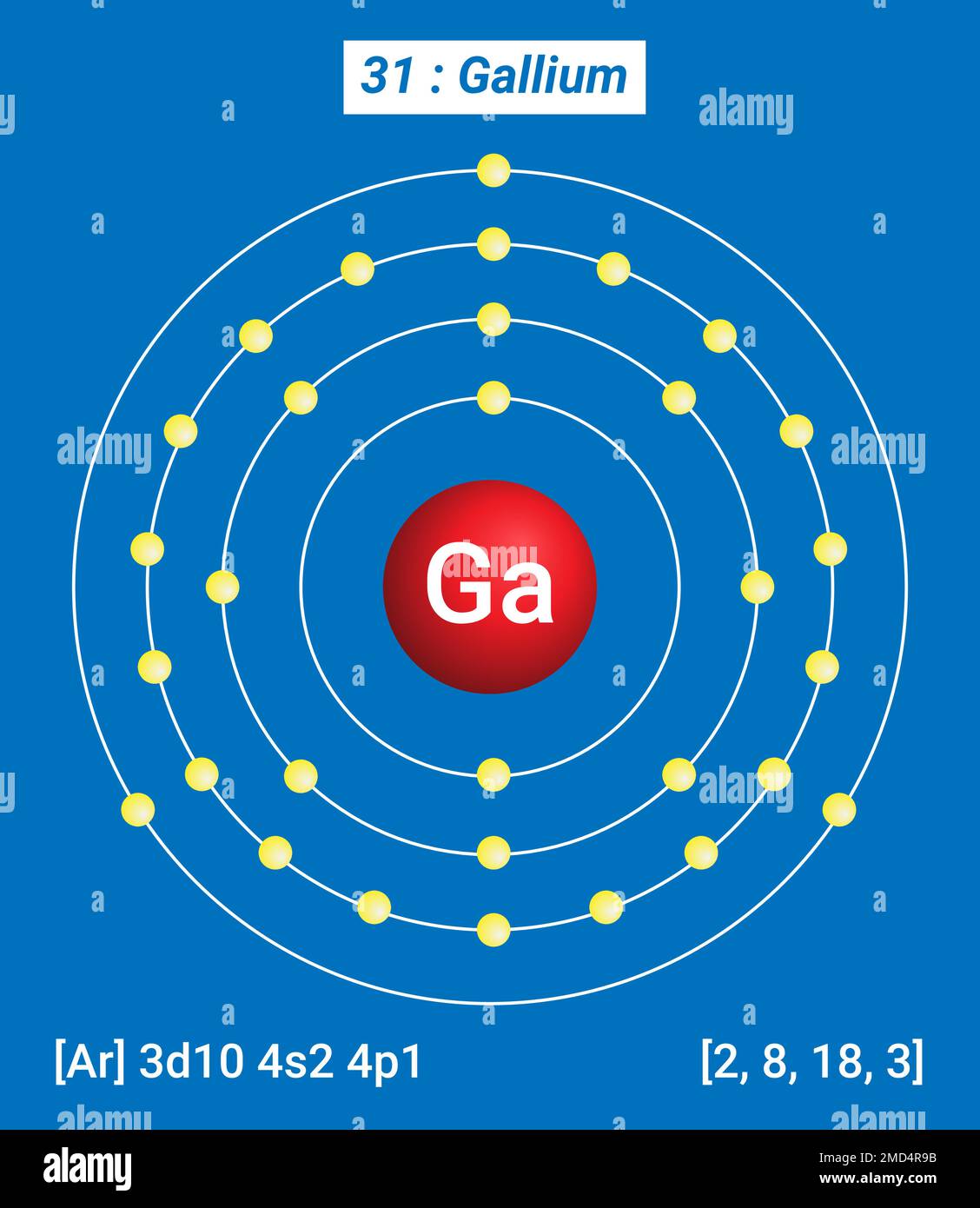
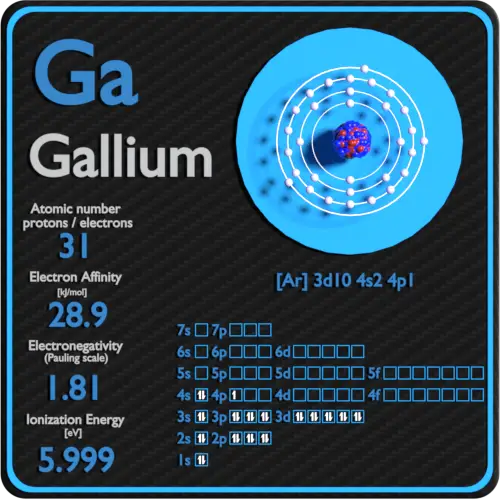
The arrangement of electrons in gallium in specific rules in different orbits and orbitals is called the electron configuration of gallium. The electron configuration of gallium is [ Ar] 3d 10 4s 2 4p 1 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

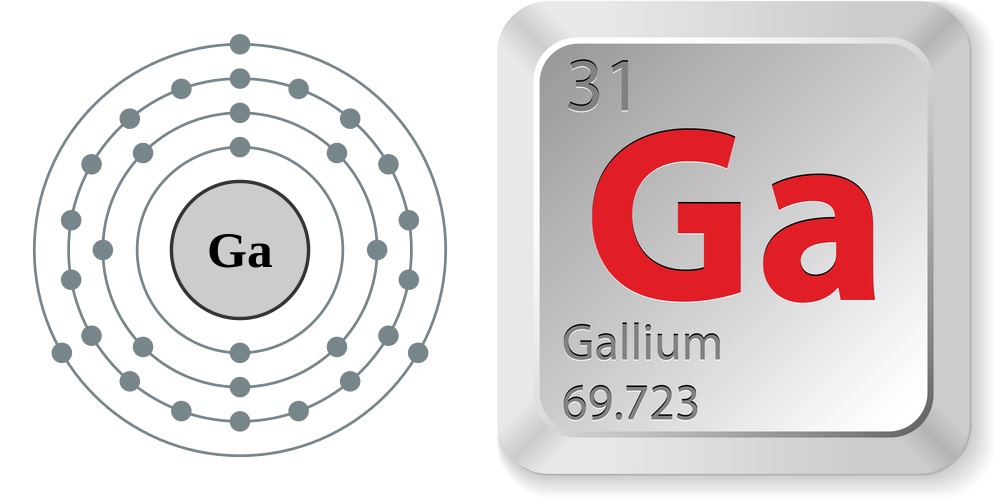
Gallium Electron configuration Symbol Atomic Number Atomic Mass
In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s and 4p subshells. There are 2 electrons in the 4s subshell and 1 electron in the 4p subshell. So gallium has a total of 2 + 1 = 3 valence electrons. Your feedback matters.

Electron Configuration for Gallium (Ga, Ga3+ ion)
Full electron configuration of gallium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 zinc ← gallium → germanium Gallium, complete electron configuration.

Gallium (Ga). Diagram of the nuclear composition, electron
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
Ga Gallium, Periodic Table of the Elements, Shell Structure of Gallium
Orbital diagram Ga (Gallium) is an element with position number in the periodic table. Located in the : 29.8 ℃. Electronic configuration of the Gallium atom. Valence electrons. Orbital diagram
Gallium Periodic Table and Atomic Properties
Referring to either Figure 2.6.3 2.6. 3 or 2.6.4 2.6. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.

Symbol and electron diagram for gallium Royalty Free Vector
Element Gallium (Ga), Group 13, Atomic Number 31, p-block, Mass 69.723. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point
갈륨 원자 구조에는 원자 번호, 원자 스톡 벡터(로열티 프리) 1916962490 Shutterstock
Electronic configuration of Gallium (Ga): Gallium is a p-block element having atomic number 31. The group number and period number of Gallium are 13 and 4 respectively. Therefore, the electronic configuration of Ga is [ Ar] 3 d 10 4 s 2 4 p 1. Suggest Corrections.
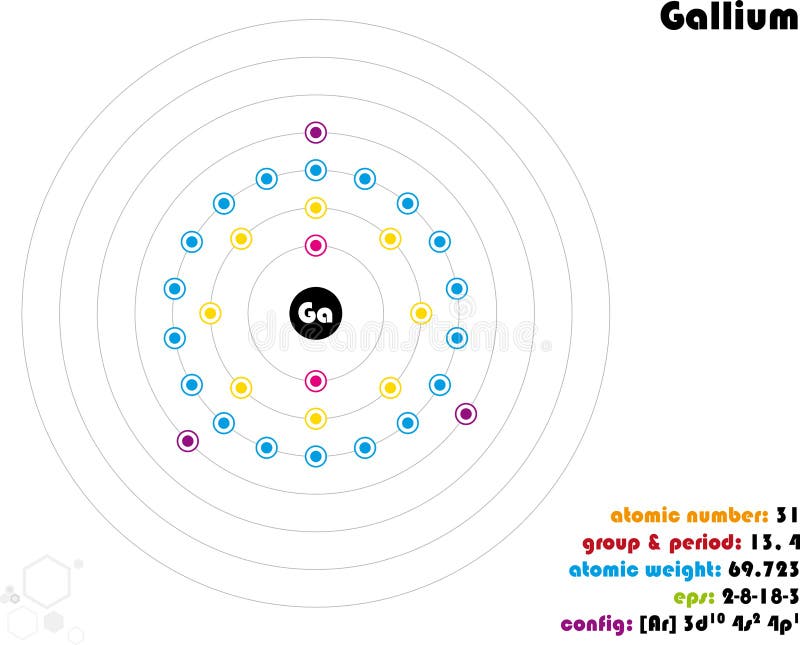
Infographic of the Element of Gallium Stock Vector Illustration of
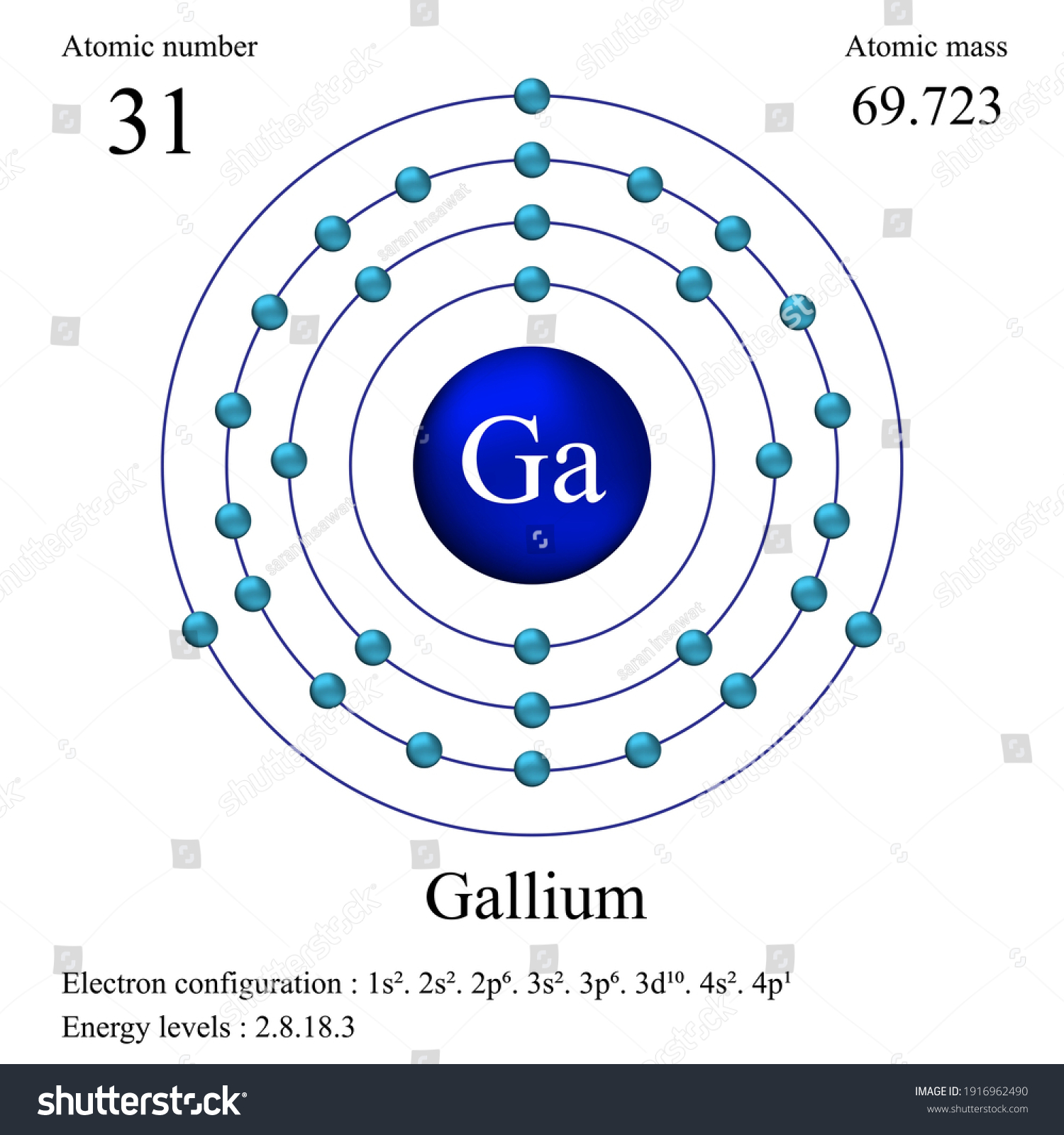
The electron configuration for Gallium, Ga is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^1 Gallium, Ga has 31 protons and 31 electrons. The superscripts represent the electrons present in each region of the periodic table. The sum of these superscripts should equal the atomic number for a neutral atom.

FileElectron shell 031 Gallium.svg Wikimedia Commons Shells
Step 1: Writing the electron shell number Gallium has 4 electron shells which are written as 1, 2, 3, 4 Step 2: Putting orbital notations after the number of electron shells Gallium atom is consists of 3 orbitals such as 's', 'p' and 'd' Step 3: Calculating the number of electron in orbitals
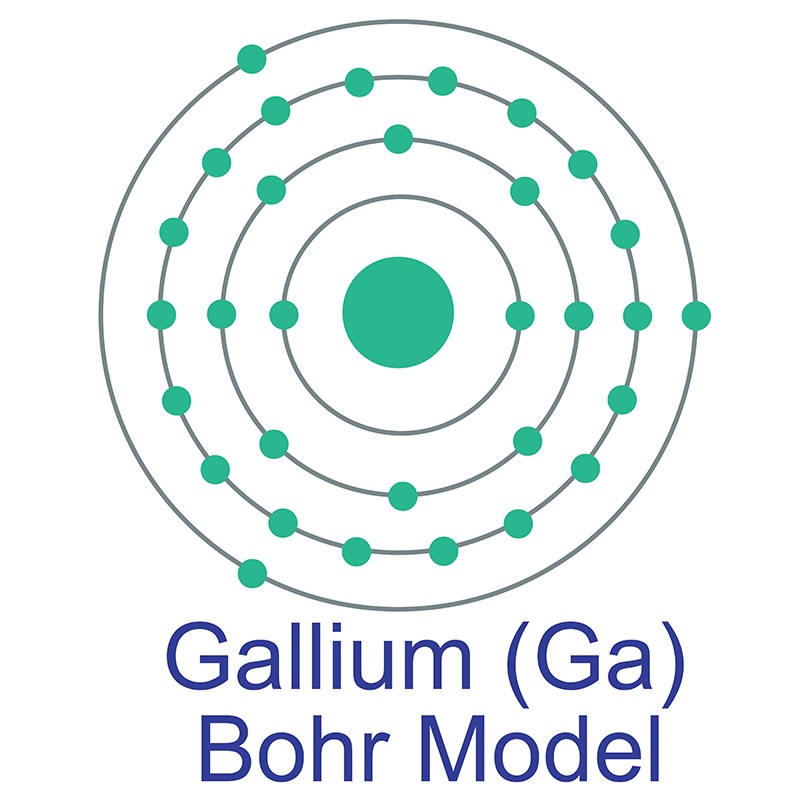
[DIAGRAM] Atomic Diagram Of Gallium
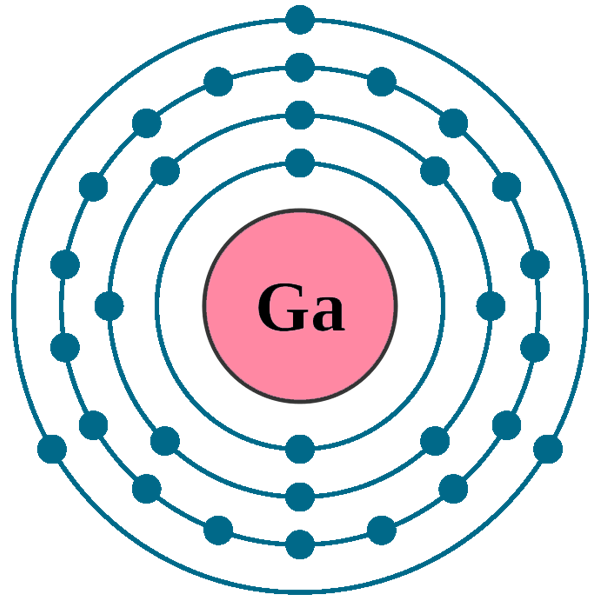
We know that gallium atoms have a total of thirty-one electrons. The electron configuration shows that there are two electrons in the K shell, eight in the L shell, eighteen in the M shell, and three in the N shell.

Ga electronic configurationHow to write electronic configuration of
Electron Configuration of Gallium: Follow the steps mentioned below to get the electron configuration of Gallium. To write the electron configuration of gallium, we should first know the total number of electrons present in a gallium atom. The gallium atom has a total of 31 electrons because its atomic number is 31 and it is a neutral atom.

Gallium, atomic structure Stock Image C023/2521 Science Photo Library
1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of gallium (Ga) is 31. Hence the gallium element has electrons arrangement 2, 8, 18, 3. This electron arrangement indicates that the outermost orbit of Gallium element (Ga) has 3 electrons.
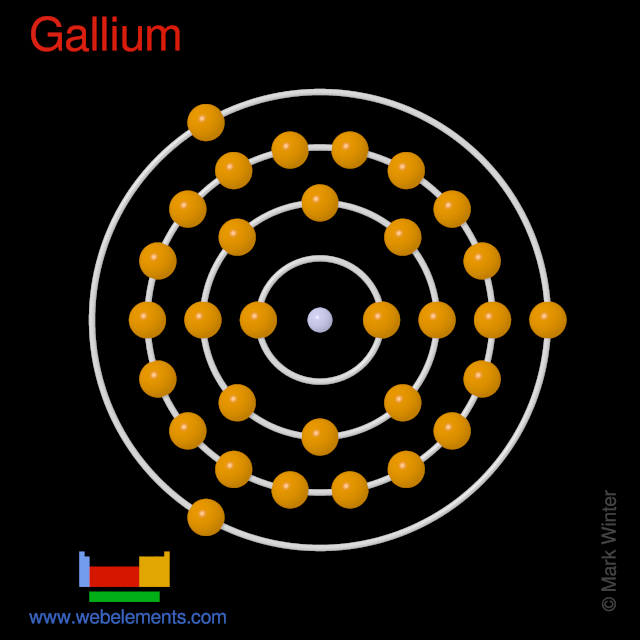
WebElements Periodic Table » Gallium » properties of free atoms
Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table. It is in the Boron family (group 13) and in period 4. Gallium was discovered in 1875 by Paul Emile Lecoq de Boisbaudran. Boisbaudran named his newly discovered element after himself, deriving from the Latin word, "Gallia," which means "Gaul.".
Facts About Gallium Live Science
The gallium atom requires five more valence electrons in its p-orbital to attain noble gas configuration. What is the complete electron configuration of gallium? The complete electron configuration of gallium is 1s2 2s 2 2p6 3s2 3p6 4s2 3d10 4p1.

A stepbystep description of how to write the electron configuration
Here, the electron configuration of gallium ion(Ga 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This gallium ion(Ga 3+) has thirty-one protons, thirty-nine neutrons, and twenty-eight electrons. Gallium ion: Protons: Neutrons: Electrons: Ga 3+ 31: 39: 28: Number of protons, neutrons and electrons for the gallium ion(Ga 3+)