
How to draw NO2+ Lewis Structure? Science Education and Tutorials
NO2 is a neutral molecule with odd electrons and can combine with other NO2 molecules and exist as a dimer N2O4. We can also write NO2 as NO2- ion as it contains a coordinate covalent bond and is called a nitrate ion. Nitrogen has one odd electron and is an example of an odd electron species. Geometry

Lewis structure of NO2. How to draw the Lewis structure of NO2. Advance
One can infer the hybridization of nitrogen in NO2 from the molecular shape of the molecule. The bent or V-shaped molecular geometry of NO2 is consistent with sp2 hybridization, as it allows for the repulsion between the lone pair of electrons and the bond pairs. V. Electron Geometry of NO2 A. Determination of electron geometry of NO2

How do you draw the lewis structure for NO2?
Geometry. NO2- Geometry and Hybridization. Nitrogen is the central atom: There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent bonds. Both oxygens get 6 electrons as three lone pairs, and nitrogen gets one lone pair: One lone pair from an oxygen is used to make a π bond with the nitrogen and thus making the ionic.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
The Nitrogen atom in the Lewis structure for NO 2 is the least electronegative atom and passes at the center of the structure. Molecular Geometry and Bond Angles of NO 2. Since the Nitrogen Dioxide (NO 2) has an extra electron in a nitrogen atom orbital, it will result in a higher degree of repulsions. However, if we consider one lone electron.

No2 Lewis Structure
Electron Geometry of NO2. The electron geometry of a molecule is determined by the arrangement of electron pairs around the central atom. In the case of NO2, nitrogen is the central atom, and it has one lone pair and two bonding pairs. According to the VSEPR model, the presence of one lone pair and two bonding pairs gives NO2 an electron pair.

NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.

NO2 Molecular Geometry / Shape and Bond Angles YouTube
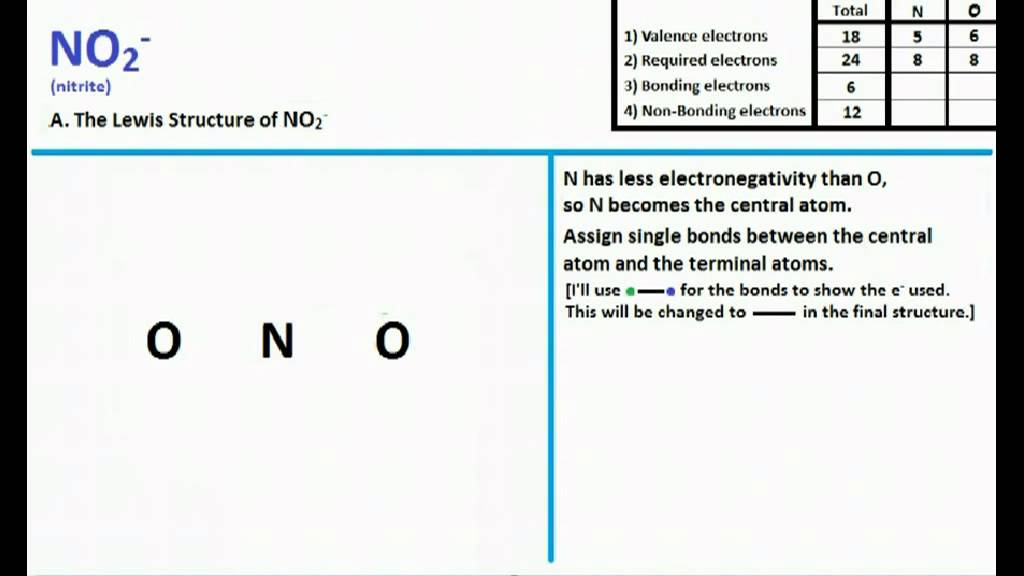
For the NO2- Lewis structure, calculate the total number of valence electrons for the NO2- molecule. After determining how many valence electrons there are in NO2-, place them around the central atom to complete the octets. There are a total of 18 valence electrons for the Lewis structure for NO2-. Nitrogen is the least electronegative atom in.
1.3 VSPER Theory The Effect of Lone Pairs Chemistry LibreTexts
A quick explanation of the molecular geometry of NO2 - (the Nitrite ion) including a description of the NO2 - bond angles.Looking at the NO2 - Lewis structur.

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
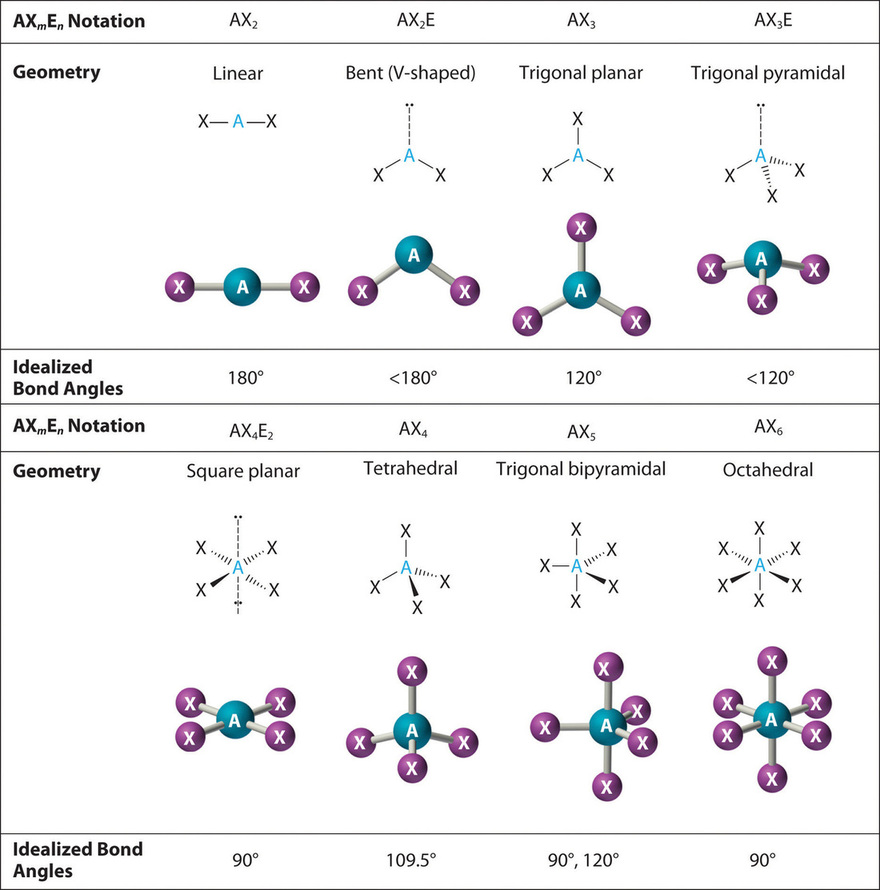
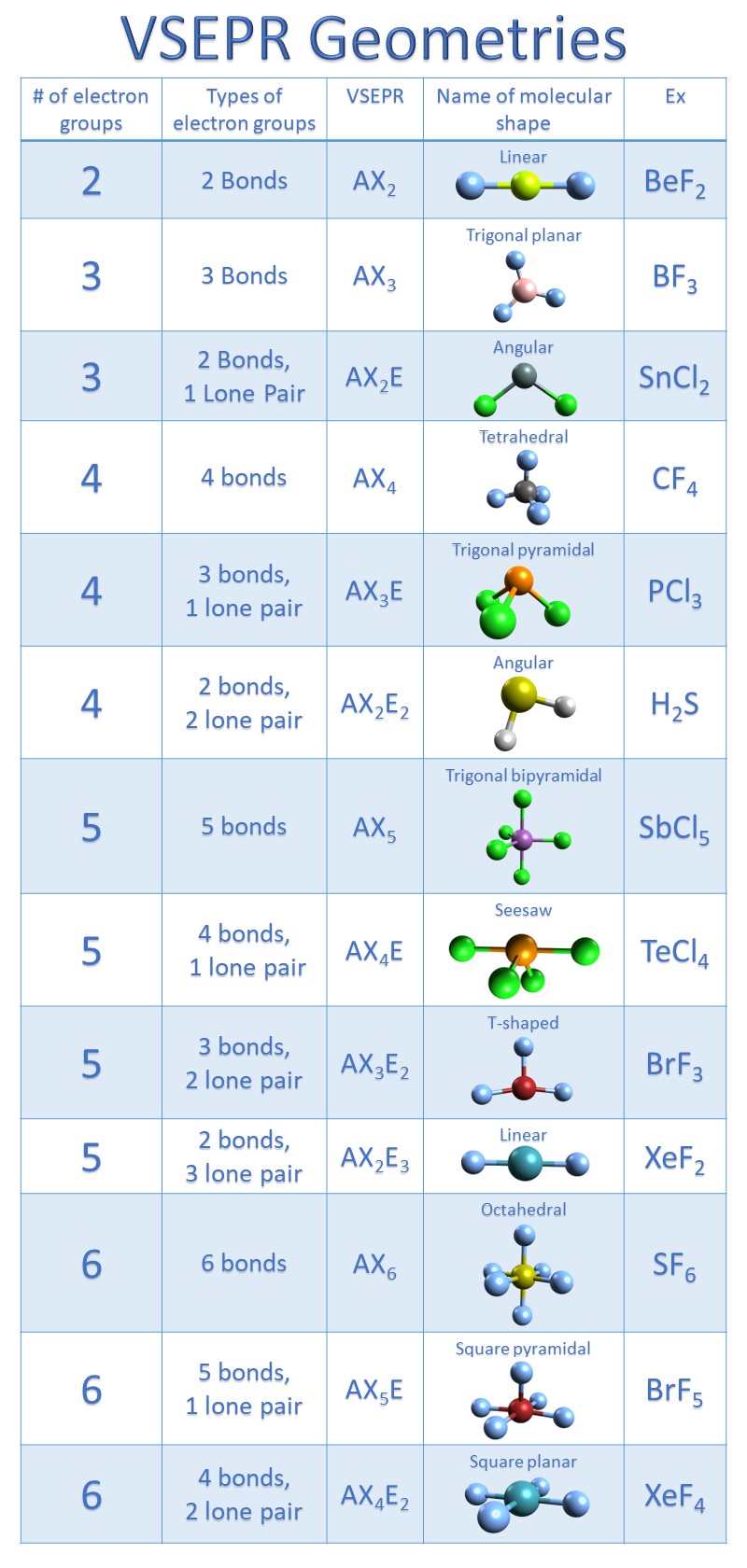
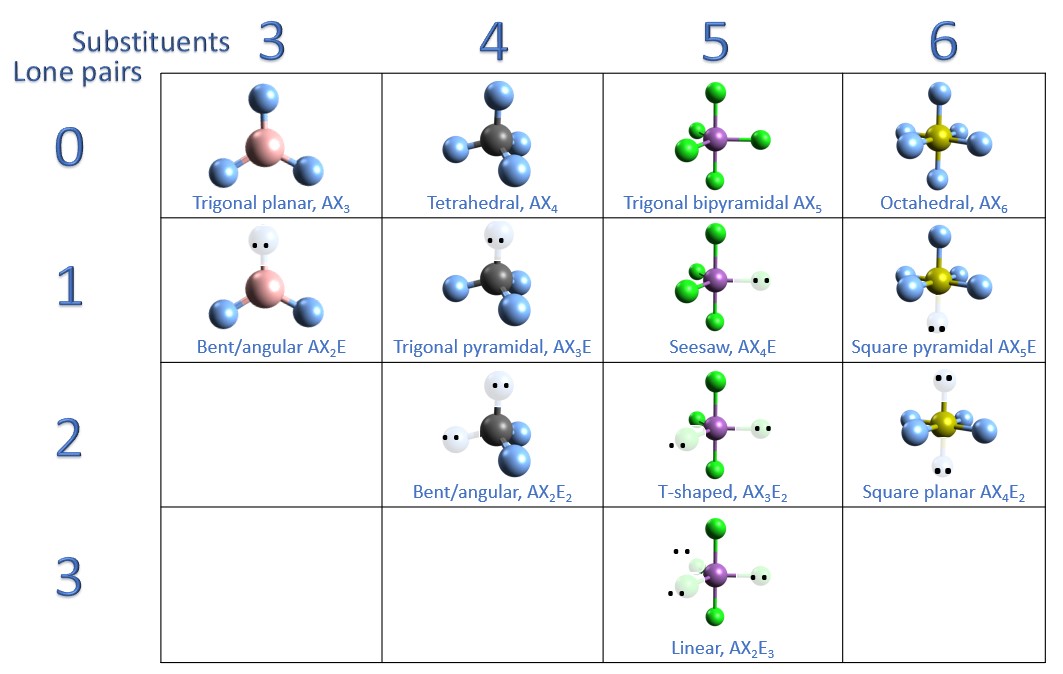
Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.
NO2 Lewis Structure and Molecular Geometry YouTube
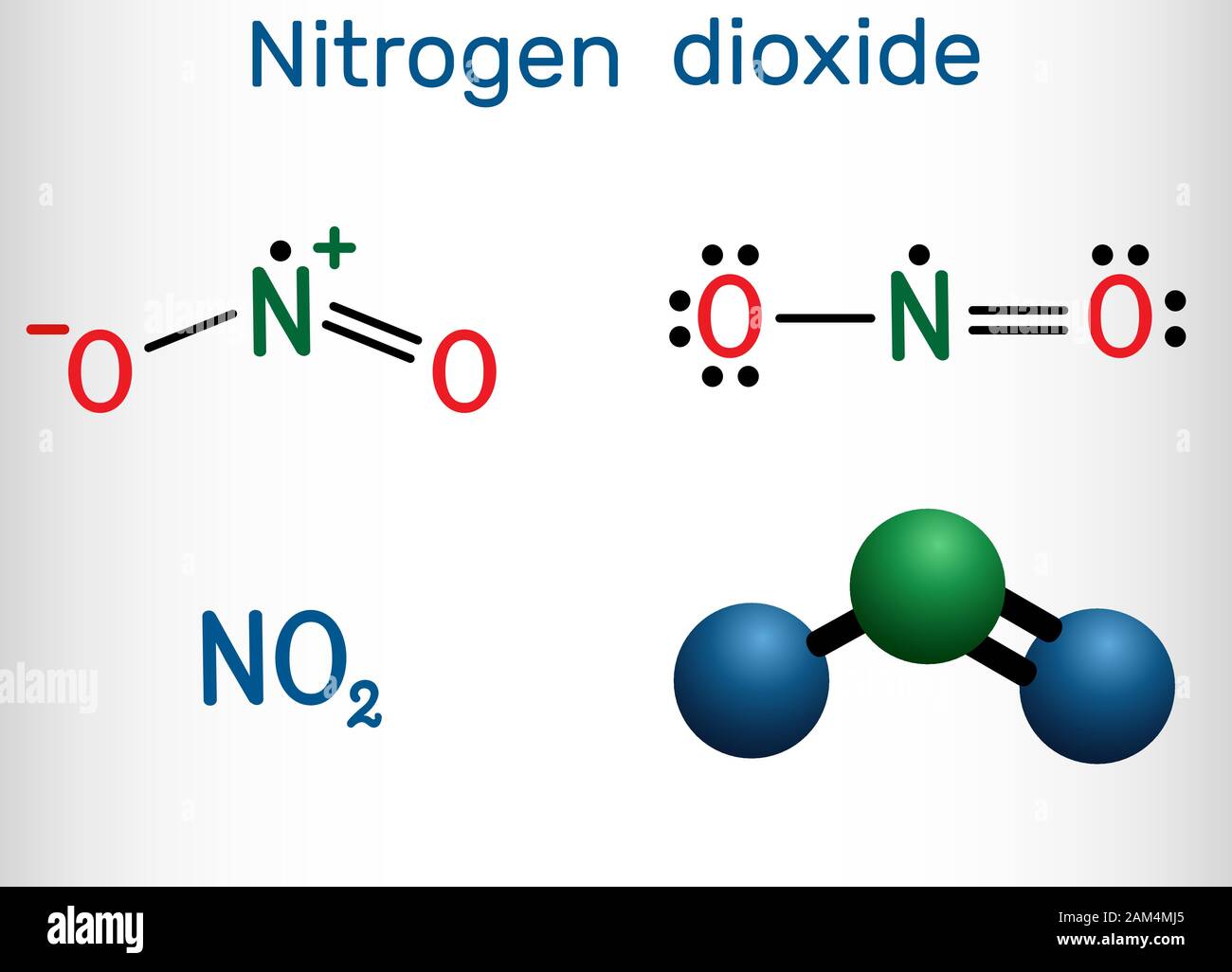
A quick explanation of the molecular geometry of NO2 including a description of the NO2 bond angles. Note the exact bond angle is 134.3 degrees).Looking at.
N2 Structure
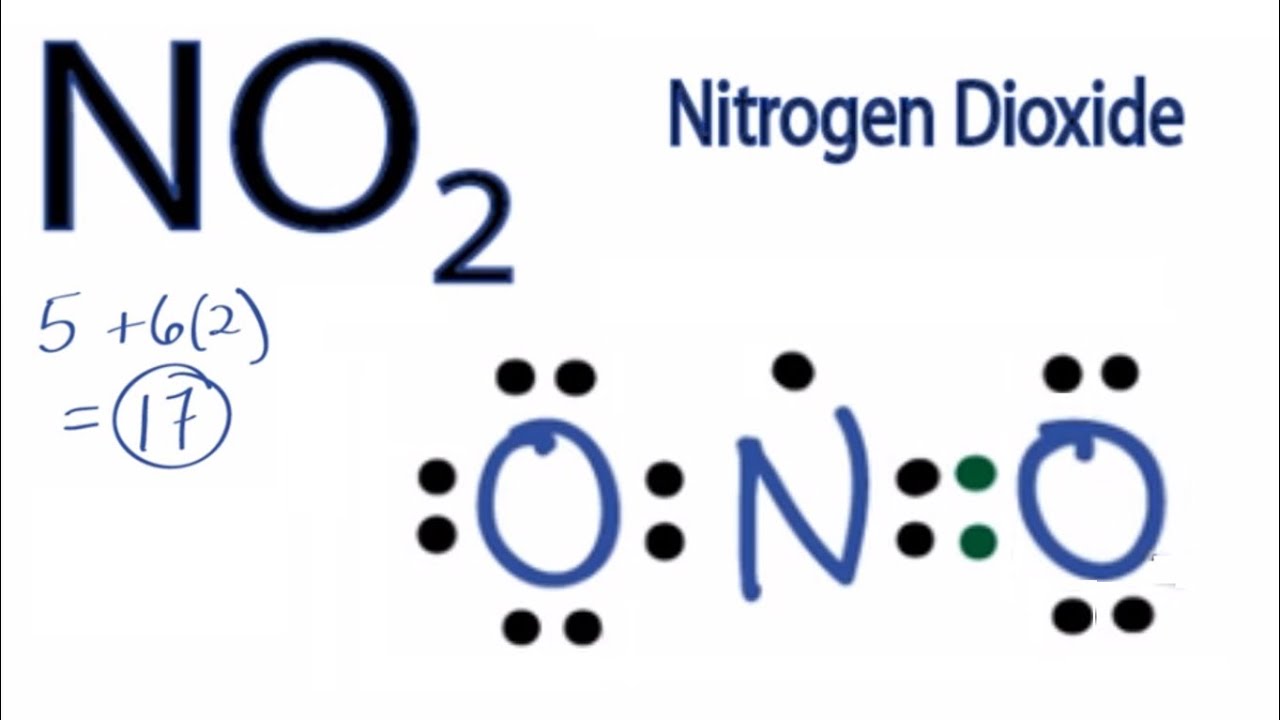
A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). The NO2 Lewis structure has a total of 17 valence electrons. It's n.

Overview NO2+ electron and molecular geometry
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: What is the Lewis structure for NO2- ? Then, what is its electron geometry, hybridization, molecular geometry, and polarity? What is the Lewis structure for NO2- ? Then, what is its electron.
Nitrogen Dioxide No2 Molecule Structural Chemical Free Nude Porn Photos
A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion).For the NO2 - structure use the periodic table to find the total number.

13+ Lewis Structure Of No2 Robhosking Diagram
Steps of drawing NO 2- lewis structure. Following steps are required to draw NO 2- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion. Total electrons pairs. Center atom selection from nitrogen and oxygen atom.
Molecular Geometry of NO2 [with video and free study guide]
The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom. This will mean that it will only have 7 valence electrons. In the Lewis structure for NO2 the Nitrogen.
Molecular Geometry of NO2 [with video and free study guide]
The total valence electrons available for drawing nitrite [NO2]- ion Lewis structure are 18. The molecular geometry or shape of NO 2- is bent or V-shaped. The ideal electron geometry of NO 2- is trigonal planar. The central N-atom has sp 2 hybridization in NO 2-. The O=N-O bonded atoms form a mutual bond angle of 134°.